Crosslinked Single-Ion Conducting Polymer Films for Use in High Performance Lithium Ion Batteries
- Student
- Morgan Seidler
- College(s)
- College of Science
- Class Year
- 2019
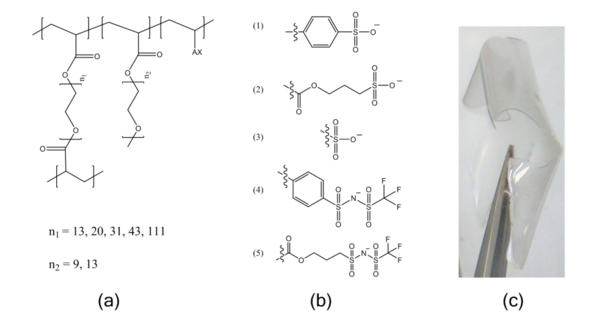 Chemical structures of (a) crosslinked ionomeric electrolytes, where A is the tethered anion and X is the counter-cation, and (b) the tethered anions, referred to as follows: (1) SS, (2) APS, (3) VS, (4) STFSI, and (5) APTFSI. A photograph of a typical crosslinked electrolyte is shown in (c)
Chemical structures of (a) crosslinked ionomeric electrolytes, where A is the tethered anion and X is the counter-cation, and (b) the tethered anions, referred to as follows: (1) SS, (2) APS, (3) VS, (4) STFSI, and (5) APTFSI. A photograph of a typical crosslinked electrolyte is shown in (c)
Solid state polymer electrolytes provide a promising alternative to existing liquid battery systems. However, the solid state electrolyte has been plagued by low ionic conductivity, which is a key measure in determining the efficiency of a battery. Varying the crosslinker length, anionic monomer, and presence of additional polymer segments does not improve the ionic conductivity sufficiently to meet the 10-3 S/cm conductivity theoretically necessary for a high performing battery system. By swelling the polymer in an organic solvent, the ionic conductivity has been shown to increase by a few orders of magnitude, making it more plausible as a replacement to the liquid battery.
In this study, the effect of organic solvents on polymer swelling ratio and ionic conductivity will be examined. I will study the transport properties of the gelled polymer electrolyte to increase the understanding of ion transport through the films, then apply the polymer to a battery system should the results be positive.